Steve Trewick
They’re cute, they’re cuddly but will they burst your bubble?
Time spent with cats is never time wasted, as Sigmund Freud is said to have said but didn’t. And a good job too because it might turn out to be poor advice in the post-COVID19 world. Evidence is rapidly accumulating to suggest that cats should probably be kept at a distance or strictly kept within your household bubble. Bluntly, you don’t know where your cat has been and cats are emerging as potential vectors of the COVID19 virus. They can be infected, get ill, exchange it with other cats and (given the origins of the disease) it is likely they could pass it on to humans. Cats within rest homes (aged residential care facilities) should be tested because there is any potential of them spreading the COVID19 virus between residents.

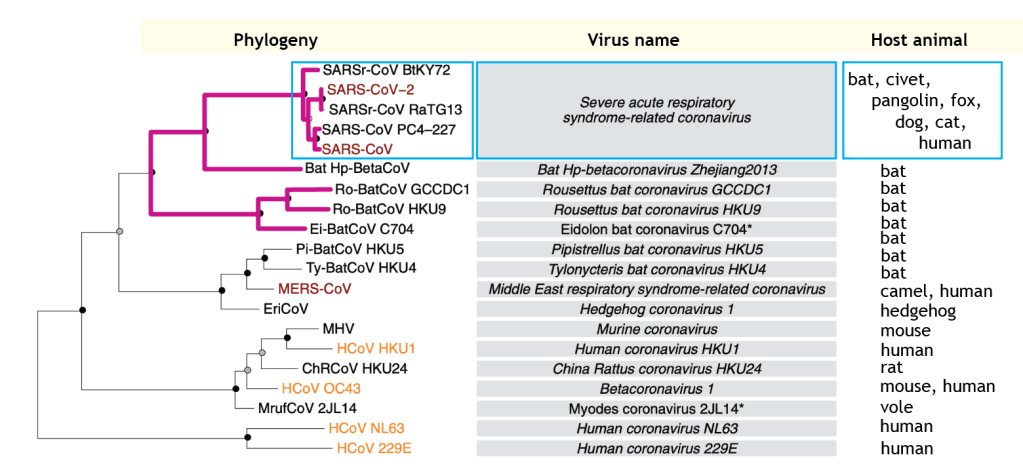
First things first though, the name of what Donald Trumps calls the ‘invisible enemy‘. The handy tag-name COVID19 that was quickly adopted around the world was coined by the World Health Organisation early this year; it means COronaVIrus Disease 2019. However, it is critical that we understand that the virus is related to other similar viruses, and so it helps to use a formal name that links related viruses together (a more robust approach set out by the WHO). The better we understand where this virus came from the better we can prepare for future pandemics (which are a certainty) and work out how to battle this one. Virus naming has special rules because of the particular ways they multiply and the rapidity with which they can evolve, and now most information comes directly from the DNA (or RNA) sequence of the virus. Adding information from COVID19 to the existing pool of viral genetic data has revealed its nearest evolutionary relatives. The virus family tree shows that the current nuisance is similar to the virus that was named SARS when it emerged in 2002. When Severe Acute Respirator Syndrome was unique the name SARS was sufficient but we now know: 1) that SARS and COVID19 are both members of the same subgroup of coronaviruses, 2) they share a common ancestor most probably hosted by bats, and 3) they are closely related to one another. Hence the decision to adopt the names SARS-CoV-1 and SARS-CoV-2 (=COVID19).
New diseases do not emerge spontaneously but result from the evolution of existing disease-causing microbes, one route to novelty in humans involves ‘zoonotic’ transfer. This involves a disease causing microbe moving to a human host from some other animal, and reflects the normal background pattern of microbes moving among potential hosts in nature. Humans are not specifically targeted by disease-causing microbes but our activities sometimes increase our exposure to potential microbial parasites; in particular the burgeoning population of humans on Earth means increased contact between species in our competition for resources. Situations where wild animals, farm animals and people interact are ideal for transfer.
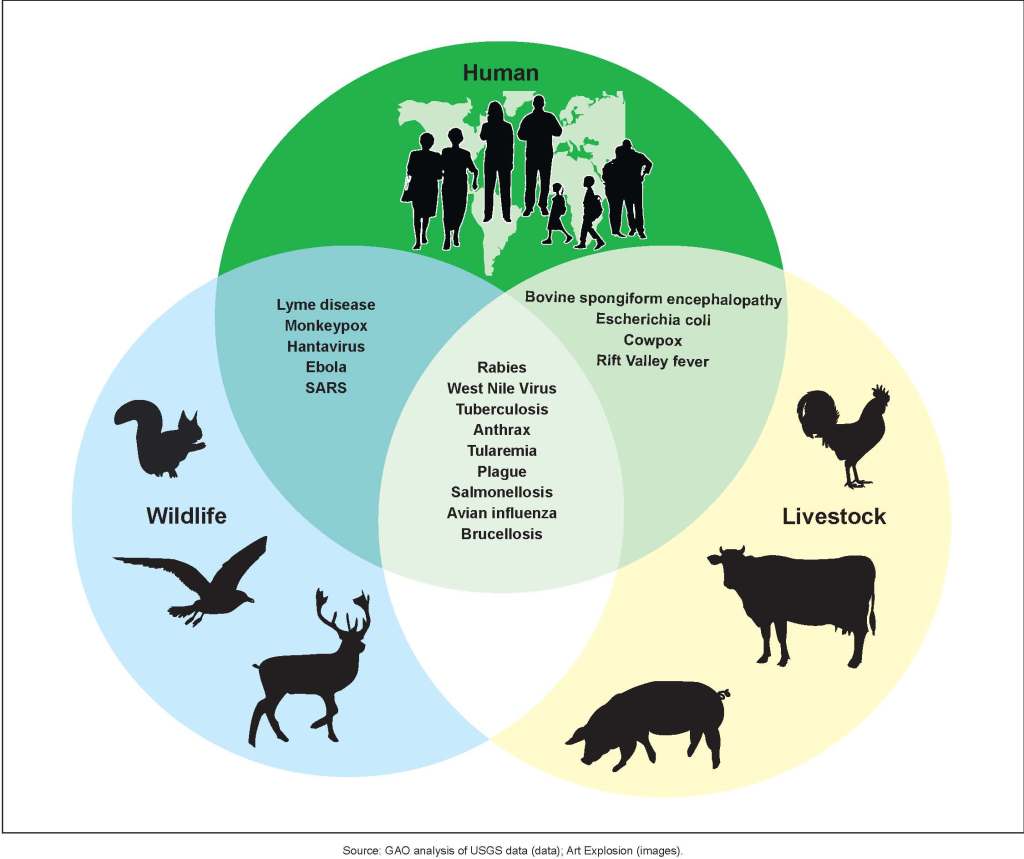
It appears that SARS-CoV-1 and SARS-CoV-2 (=COVID19) are descended from viruses transmitted amongst bats, but that does not mean a human caught the disease directly from a bat. In fact, we do not know all the different animals that can be infected by these virus; we have not had time to test extensively among humans for SARS-CoV-2 let alone other species. Current evidence indicates that the intermediary might have been another wild mammal traded in a Chinese market. Genetic samples from animals in markets show a high prevalence of SARS-CoV in masked palm civets Paguma and other carnivores including racoon dog Nyctereutes, fox Vulpes, mustelids Melogale, and cats Felis. Significantly, although there is high SARS-CoV incidence among civets in markets, wild-living civets that have been tested are free of infection. This supports the idea that zoonosis is associated with human activity.

To invade a host cell, so it can replicate, a virus needs to connect to a particular cell protein in the host. For SARS-CoV-2 this is a blood pressure-regulating protein called ACE2. The ACE2 protein in human cells to which the so-called ‘spike protein’ of SARS-CoV-2 attaches (allowing it entry to human cells) is universal among vertebrates. In fact, the ACE2 protein of humans, several other primates, and domestic cats are nearly or completely identical. This means SARS-CoV-2 (=COVID19) can break into cat cells as well as human cells where ACE2 is expressed (especially the lungs). Human SARS-CoV-2 can still bind to palm civet ACE2, despite the genetic mutations that distinguish palm civet SARS-CoV that gives SARS-CoV-2 access to humans.
Having near identical ACE2 protein means there are many species of mammal that can host SARS-CoV-2, not only humans, but also domesticated cats, dogs, tigers, ferrets and many others. Although there is some evidence for transmission via dogs in the Italian outbreak, domestic cats have shown a greater potential to spread SARS-CoV-2 because they are more easily infected and roam widely. The behaviour of domestic cats in approaching other cats and new people increases the chances of them acting as disease vectors. Domestic cats are so susceptible to SARS-CoV-2, that it has been recommended that “Surveillance for SARS-CoV-2 in cats should be considered as an adjunct to elimination of of COVID-19 in humans.”
During Lockdown level 4 in New Zealand we have focused on people socially distancing and maintaining their small household “bubble”. Meanwhile most cats have roamed and continue to roam uncontrolled. There are more than one million domestic cats in NZ with 44% households providing homes to an average of 1.5 cats each, most of which roam outdoors. Many rest homes for the elderly have a resident cat, or promote therapy using contact with cats. Households with children are the most likely to have companion animals and in the majority these are cats, which in suburbs roam across an average of 16 neighbouring properties (= 16 social distancing bubbles). A study in Wellington city where the average house section is 600 square metres, found cats roamed on average over 3 hectares. That is the equivalent of 50 city sections, but roaming depends on the cat and the location, and one cat roamed over 200 hectares.
Swelling the domestic cat population in New Zealand are an estimated 2.4 million feral cats roaming under the radar in urban and rural environments. Little is known about the contact between domestic and feral cats in New Zealand. Globally there is a long list of zoonotic diseases associated with cats, and the role of free-roaming cats is well recognised.
As yet cats have not been tested in New Zealand but pet cats in New York and Belgium have tested positive. In Wuham China 15% of cats tested had antibodies for SARS-CoV-2, showing that they had recovered from the disease. No link has been found between the SARS-CoV-2 hospitalisation of cat-loving UK prime minister Boris Johnson and Larry the cat who roams in and out of the No.10 bubble. But the implications are clear. Cats are susceptible to SARS-CoV-2 and can transmit it to one another and experience shows that human–animal transmission of viral diseases are significant. MERS-CoV for instance is known to be transmitted to people by close contact between camels and people and the pool of genetic diversity within the related MERS-CoV is shared among human-camel community.

The success of a viral strain is measured only in its survival. As a surviving host individual will usually eventually develop some immunity to a particular infection, viral survival requires a large and accessible pool of susceptible hosts, so there is a selective advantage to being able to invade different species. This means that viral (and bacterial) pathogens will continue to cross species boundaries and pandemics in the most abundant, widespread and mobile mammal species on Earth is inevitable and probably devastating.


Great article, I did wonder about cats as our council has been saying dogs need to be kept in your own bubble but cats get to roam freely. I wonder what rules will be put in place for cats.